Antibody Tests Are Still A Long Way From Holding The Key To Ending COVID-19

- Novel coronavirus antibody tests can help determine whether a person has contracted COVID-19.
- COVID-19 antibodies are also being studied to determine if they could hold the key to potential treatments or vaccines.
- Right now, reliable COVID-19 antibody tests are not widely available, though many are currently in development.
Back in mid-March, the coronavirus got me—in a subtle sneak attack. My symptoms were not the hallmarks of the COVID-19 infection. There was no cough, no fever, no chills. Instead, I had moderate body aches that disrupted my sleep one night and a few days later, a blazing heachache. It wasn’t until I lost my sense of smell—a late-breaking (and now telltale) sign of the virus—that I thought, “Uh oh.”
I live in New York City, the infamous epicenter of the novel coronavirus in the U.S., but where diagnostic tests are in maddeningly short supply. The full scope of that shortage has yet to come into full view: The Covid Tracking Project measures how many people have been tested nationally, but there is no way to count the total number of people who wanted or needed tests, but couldn’t get them.
All we know is that the number of people who have been sick has likely been vastly undercounted. In fact, one study that has yet to be peer-reviewed, estimates that the US may have at least 100 times the number of COVID-19 cases than have been accounted for. And judging by preliminary research from China that suggests a 30 percent chance of a false negative result, diagnostic tests may not always be accurate, even when you can get your hands on one.
So, I followed the directive to #stayathome and not visit an emergency room, given that I don’t have any underlying conditions and I’m not in a high-risk group. There, I rode out the relatively mild discomfort in isolation. (To be honest, my biggest battle was with the low-key dread that the symptoms would get worse.) Gratefully, they didn’t and 10 days later, I felt pretty much back to normal.
Are antibody tests the answer to the COVID-19 pandemic?
Now, many weeks into the pandemic, talk has turned to a different kind of test—one that looks for antibodies that indicate you’ve had the COVID-19 virus (the clinical name for which is SARS-CoV-2). I, for one, was anxious to know.
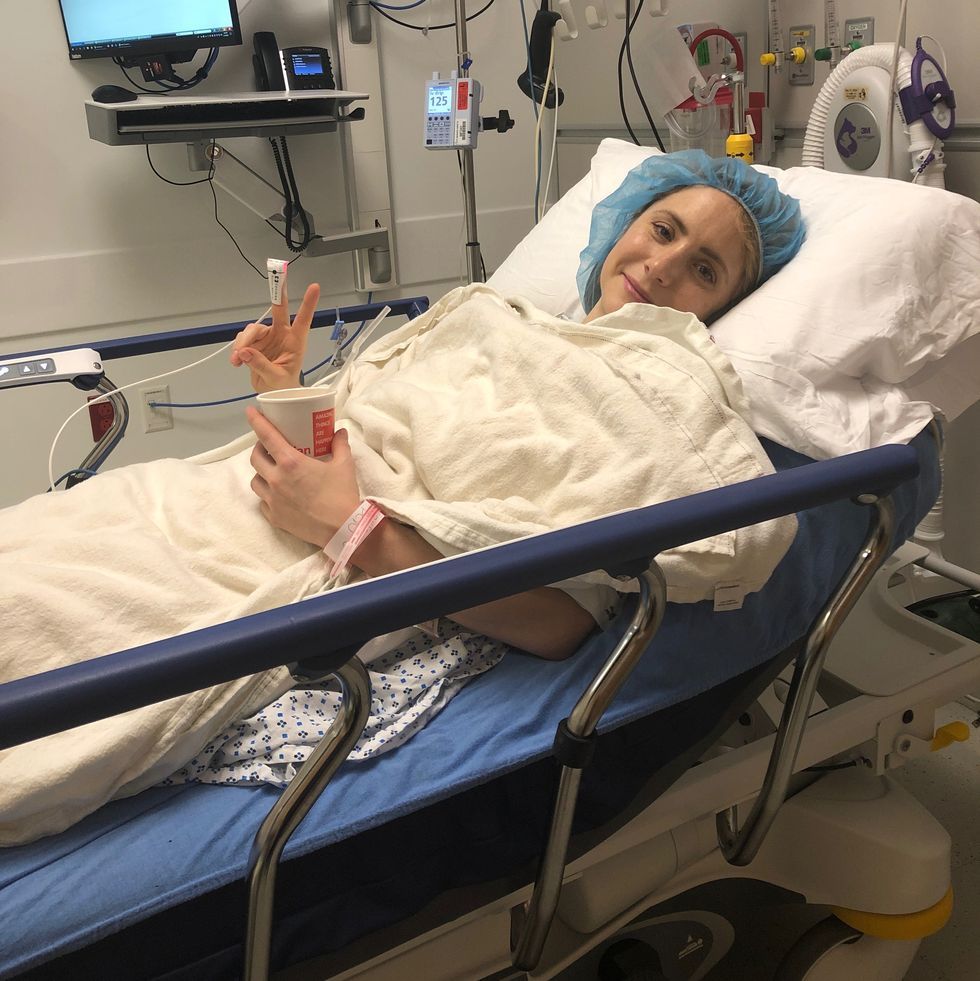
So when I heard through a friend that a medical practice in New Jersey was facilitating testing, I made an appointment and drove myself out there in search of peace of mind. I received a serologic test, requiring a simple, speedy blood draw. The lab fee cost $149, and was not covered by my insurance. The results came back a few days later… positive for SARS-CoV-2 antibodies.
I went over the results with Dr. AnnaLisa Pastore, MD, founder of Chi Medicine, who ordered the test, and she broke down the different markers for me: “The igM antibodies are the acute ones that are produced when you first get sick, the igG antibodies are the ‘memory’ antibodies that will potentially provide longer-term immunity, and the igA antibodies are ones that live in the mucosal membranes of the gut and respiratory tract,” explained Dr. Pastore. I was positive for all three markers.
But there’s so much more to unpack when it comes to these nascent tests.
What can you learn from a COVID-19 antibody test?
For starters, an antibody test should (operative word) conclusively tell you if you have or haven’t had COVID-19, based on whether antibodies are present. (You develop them within a week to 20 days of being infected, so testing too early can result in a false negative.)

Knowing whether you have antibodies can “help identify those who have potentially developed immunity and can return to work as well as those who don’t have antibodies who might be susceptible to infection and/or need a vaccine,” says Hari Krishnamurthy, a lead scientist for Vibrant America, the science and technology company that developed the test I was given.
Dr. Pastore notes that antibody tests could also help inform when it’s appropriate to integrate back into public life. (Dozens of public health experts have gone on record saying that accurate, large-scale testing is necessary before we can end the national quarantine period).
Dr. Pastore also believes there could be a psychological benefit to getting tested, particularly for those who have recovered well. “Knowing you’ve successfully fought the infection can alleviate fear and give you more confidence in your immune system’s ability to function,” says Dr. Pastore.
Can antibodies unlock the key to a vaccine or treatments for COVID-19?
The American Red Cross has partnered with the FDA to evaluate whether the plasma of people who have recovered from COVID-19 is an effective treatment for those suffering from severe infections, or for those who are at risk for progressing to a life-threatening condition. (Back in late March, a hospital in Houston became the first to use a blood transfusion to treat a critically ill patient, but doing so is still an experimental therapy.)
Aside from providing confirmation of infection, antibody testing could be useful for identifying potential plasma donors.
Other therapeutics and targeted medications are in development and some of them harness the use of antibodies to attack the virus. But experts say that coming up with an effective one-size-fits all treatment may be out of reach, particularly because scientists don’t yet fully understand how genetics and other factors play into the wide spectrum of outcomes we’re seeing—from asymptomatic and mild cases to severe respiratory disease and death.
It’s also important to know which combination and concentration of antibodies could trigger the cytokine storm (an over reaction of the immune system) that can kick-off a devastating inflammatory response in certain people.
As for a vaccine, there are over 100 COVID-19 vaccines in development and there are likely to be a handful that make it to market. But that is probably a year away and the process is fraught with guesswork.
One aspect of the COVID-19 virus that is being monitored closely is its mutability and how that will impact the development of a successful vaccine. (So far, this virus has reportedly mutated eight times.) How well antibodies interact or bind to these mutations determine how often a vaccine needs to be updated, from never (like the measles vaccine) to annually (like the seasonal flu vaccine).
How accurate are the antibody tests and where can I get one?
In general, the thinking is that antibody tests will be much more widely available than the coronavirus diagnostic tests—but we’re not there yet. A number of serologic antibody tests are in development around the globe. The FDA approved several tests, but also issued guidance allowing antibody tests to be manufactured and distributed without authorizations.
In the wake of those loosened regulations, more than 90 companies informed the FDA that they were selling antibody tests—without submitting validation data that shows they actually work. And many seem to be plagued with issues.
Case in point: British officials paid $2 million for two different antibody kits that didn’t work. One complicating factor: Some of the tests may not be specific enough to the new COVID-19 coronavirus and instead may pick up a signal from antibodies made in response to infections from former coronaviruses that cause common colds. What’s really troubling is that false positives could lull us into thinking we’re in the clear—when we’re not.
It is also unclear whether health insurance will eventually cover antibody testing.
Does having antibodies mean you have immunity?
“If you have the antibodies it means you have mounted an immune response to the virus,” states Jayaraman. “People who have resolved the symptoms of the infection and have an antibody response are likely immune to the infection, but more studies are needed.”


Epidemiologist and infectious disease specialist Marc Lipsitch, wrote an op-ed for the New York Times, theorizing that “after being infected with SARS-CoV-2, most individuals will have an immune response, some better than others. That response, it may be assumed, will offer some protection over the medium-term—at least a year—and then its effectiveness might decline.”
Another thing to determine: Whether mild symptoms equate to mild immunity, meaning if you didn’t get really sick then your antibody (and therefore, immune) response may be weaker, while still being protective against a severe secondary infection. So many questions!
The big hope is that SARS-CoV-2 will act like viruses such as chickenpox and that antibodies will trigger life-long immunity. In that case, herd immunity would stomp out the spreading, and (coupled with vaccination) eventually end the disease itself.
Will antibody testing end social distancing?
The most pressing question on everyone’s mind: When can we get back to normal? Or at least a new normal. As reported by CNN, former FDA Commissioner Dr. Scott Gottlieb cited antibody testing as a crucial part of reopening the country in an action plan he helped devise. And Dr. Anthony S. Fauci, the infectious disease expert seen at White House briefings, has floated the idea of “certificates of immunity” or so-called “immunity passports” as a way to phase out the lockdown.
At the risk of stating the obvious, I know how lucky I am that I was able to locate a testing location thanks to a referral from a health practitioner friend, that I own a car and was able to drive myself there, and that I could afford to pay the lab fee to process the test. It wasn’t about jumping to the front of any lines as much as it was about discovering the ability to get the test and pursuing it—both for my own curiosity and to inform this article.
Since receiving the test, I haven’t changed my behavior: I still practice social distancing, wear a mask out in public, and try to do as many things as I can to boost my immunity. As Dr. Pastore says: “Whenever you get information, it’s about how you use it. The goal is to be empowered to make better decisions—for yourself, but also in the best interest of those around you.”
Source: Read Full Article