Altered microbiome after antibiotics in early life shown to impact lifespan

A team of researchers from SAHMRI and Flinders University has found a link between the type of microbiome that repopulates the gut following antibiotics and shortened lifespan in mice.
The study recently published in Cell Reports, is the first of its kind to examine the long-term effects of exposing normal healthy mice to antibiotics in early life, tracking the mice from infancy to old age, 102 weeks later.
Professor David Lynn, an EMBL Australia Group Leader at SAHMRI and Flinders University, who led the project, says that previous studies investigating the causative link between gut microbiota and lifespan have largely been restricted to invertebrates and mice with shortened lifespan due to genetic deficiency.
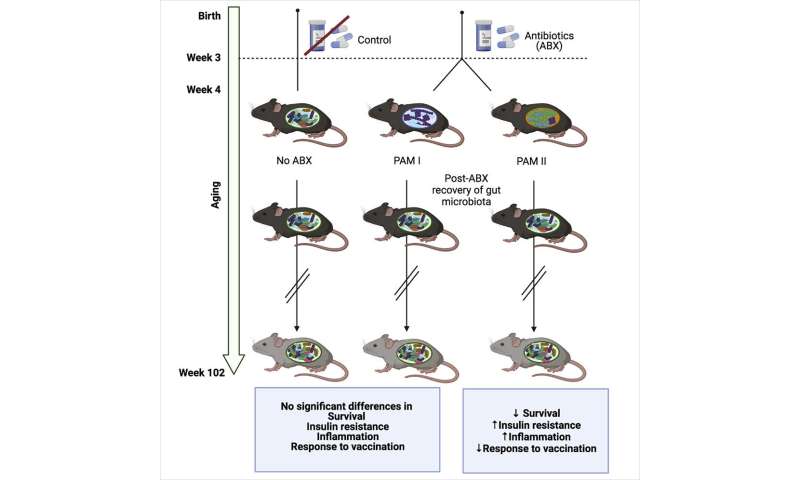
“This is the first time we’ve been able to monitor the impact that changes in gut microbiome due to antibiotics in early life can have on mice throughout their normal lifespan,” Prof Lynn said. After microbiome diversity was severely depleted following antibiotics, researchers found that the gut was repopulated with either one of two different dominant types of microbiota, which they called “PAM I” and “PAM II.”
“Mice with PAM II microbiome displayed increased insulin resistance in later life, indicating metabolic dysfunction, as well as significantly higher levels of inflammation in multiple different tissues including the blood, liver and brain,” Prof Lynn said.
“The PAM II mice died at approximately double the rate compared to those that were repopulated with the PAM I microbiota, even though both groups were littermates exposed to the same antibiotics.”
These effects were observed even though the composition of microbiota in both groups of antibiotic exposed mice returned to normal in the weeks after antibiotic exposure and long before the detrimental effects on metabolism and inflammation were observed in the PAM II mice.
“Our data suggests that the type of microbiome that repopulates the gut after antibiotics has the potential to reprogram the mammalian immune system with long-lasting effects including on longevity,” Prof Lynn said.
Previous studies in humans have linked antibiotics to a litany of long-term health effects; but have often shown varying results between different individuals and different studies.
Prof Lynn says this latest study suggests that this variability may be due to the type of microbiome that recolonises after antibiotics, rather than the depletion of the microbiome during antibiotic exposure.
To further prove the results were due to differences in microbiome recovery and not antibiotics, the researchers took the extra step of colonizing mice with no microbiome, known as “germ-free,” with the PAM I and PAM II microbiome.
These experiments showed changes in the immune system of germ-free mice colonized with the PAM I microbiota compared to the PAM II microbiota.
Prof Lynn says that those with PAM II typically had a more inflammatory immune system.
“These data suggest that differences in the microbiota following antibiotics in early life can reprogram the immune system long-term, with the consequences of this reprogramming emerging later in life, including effects on immunity, metabolism and even lifespan,” Prof Lynn said.
Source: Read Full Article