A link found between mutated DNA and a rare blood condition

Acquired aplastic anemia (AA) is a rare blood disorder that causes the individual to have suppressed levels of hematopoietic stem progenitor cells, which ultimately mature into various types of blood cells. In many individuals with this disorder, this results in bone marrow failure. Scientists have examined the molecular and genetic facets of blood cells from AA patients in an attempt to understand the biological mechanisms that cause this disease.
One important set of genes in the human immune system is the human leukocyte antigen (HLA) system. HLA genes encode cell surface proteins that help regulate immune cell activities. Previous works have demonstrated that blood cells from some AA patients lack certain types (alleles) of the HLA-A and HLA-B. Now, researchers at Kanazawa University have identified a novel common mutation that frequently occurs in people suffering from AA. In a new study published in Haematologica, the group shows that these HLA-A and HLA-B alleles contain the common nonsense mutation, which is a change in the gene’s DNA sequence that causes the blood cells to stop making the HLA protein prematurely.
Many scientists believe that further clarifying the loss of these HLA proteins on the surface of certain blood cells will allow physicians to better diagnose and treat individuals with AA, as well as those with other causes of bone marrow failure. While there is some understanding of specific mutations in this group of HLA genes, a large majority of AA patients do not have them. To identify additional mutations that cause HLA loss, the researchers used a technique called next-generation sequencing.
“This sequencing approach is fast and extremely sensitive,” says Hiroki Mizumaki, lead author of the study. “It tells us the sequences of the HLA genes in blood cells from each AA patient. We can compare them with each other and find a novel mutation.”
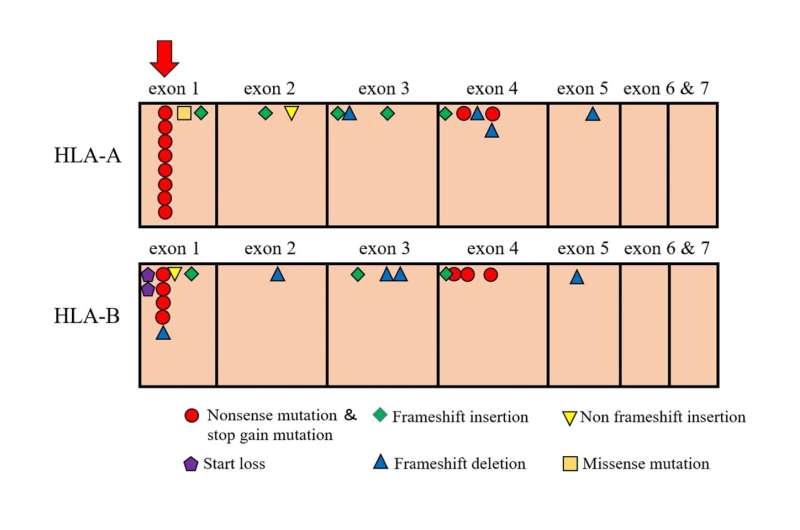
During their analysis, the team found a common mutation at codon 19 in exon 1 of these HLA genes. The DNA base at this location should be “C,” but was a “T” in these individuals. This sequence change seems minor, but it sends an incorrect message to the cell and tells it to stop producing this HLA protein.
“We found this mutation present in the DNA of 29% of the AA patient samples that we examined,” explains Shinji Nakao, senior author. “Interestingly, we observed it only in four different HLA-A alleles and in eight separate HLA-B alleles.”
Because the mutation in exon 1 was seen in numerous alleles, the results are very biologically relevant and significant. Notably, of the 45 AA patients whose DNA contained this mutation, 37 showed a positive response to immunosuppressive therapy. “This correlation between the HLA exon 1 mutation and AA patientresponse to therapy was an extremely exciting discovery,” says Mizumaki.
Source: Read Full Article