A bioinformatics approach for detecting SARS-CoV-2 co-infections

In a recent study published on the medRxiv* preprint server, researchers discuss a novel bioinformatics-based method for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) co-infections with the Delta and Omicron variants.
Study: Detection and prevalence of SARS-CoV-2 co-infections during the Omicron variant circulation, France, December 2021 – February 2022. Image Credit: NIAID
Background
To date, five SARS-CoV-2 variants of concern (VOC) have been identified based on their transmissibility and immune escape capability. The emergence of SARS-CoV-2 VOCs has caused the coronavirus disease 2019 (COVID-19) pandemic to persist and continue to cause significant impacts to both the mental and physical health of people around the world.
The SARS-CoV-2 Delta variant, for example, was particularly threatening to public health as a result of its high transmissibility and ability to invade the host immune system. Although both Delta and Omicron share several mutations in their genome, the Omicron variant has a significantly greater number of specific mutations, especially in its spike (S) gene.
The detection of these variants through whole-genome sequencing (WGS) is obstructed by several problems, including variations in primer efficiency with respect to the target regions in various variants. Additionally, the sample handling process and absence of validated bioinformatics tools to detect co-infections can also prevent the accurate identification of variants responsible for a new case of COVID-19.
About the study
In the current study, Delta and Omicron variants were isolated from nasopharyngeal swabs, sequenced to confirm their strain, and subsequently cultured in the Vero E6 TMPRSS2 cell line. Eleven different Delta:Omicron ratios (0:100, 10:90, 20:80, 30:70, 40:60, 50:50, 60:40, 70:30, 80:20 90:10 and 100:0) with similar loads were then established to simulate various coinfection scenarios.
Isolate sequences were acquired by four different sets of SARS-CoV-2 primers. Simultaneously, libraries were generated with the COVIDSeq-Test, and samples were sequenced with 100 base pair (bp) paired-end reads using the NovaSeq 6000 Sequencing system.
In addition to the simulated co-infections, the researchers were also interested in identifying the prevalence of co-infections in a total of 21,387 random samples. These samples were collected between December 2021 and February 2022 from three distinct sample groups, of which included out-patients of Flash surveys, hospitalized patients of Flash surveys and Respiratory Viruses of Hospices Civils de Lyon (HCL), and healthcare workers of HCL.
An unbiased bioinformatics method was applied to aid in the detection of these co-infections. Briefly, the sequenced reads were processed using bioinformatic pipeline seqmet. For obtaining paired reads without adapters, they were cut using cutadapt software and aligned with the reference genome of SARS-CoV-2, and re-aligned for improving detection sensitivity.
Study findings
In their initial experiments on simulated co-infections, the researchers found that over 90% of Delta-specific mutations were identified in all mixes with the four primer sets. Comparatively, between 27% and 78% of Omicron-specific mutations were detected in the 90:10 mix. Taken together, these experiments confirmed the performance capabilities of four different sets of primers that were subsequently used for detecting true co-infections between different SARS-CoV-2 lineages in random samples.
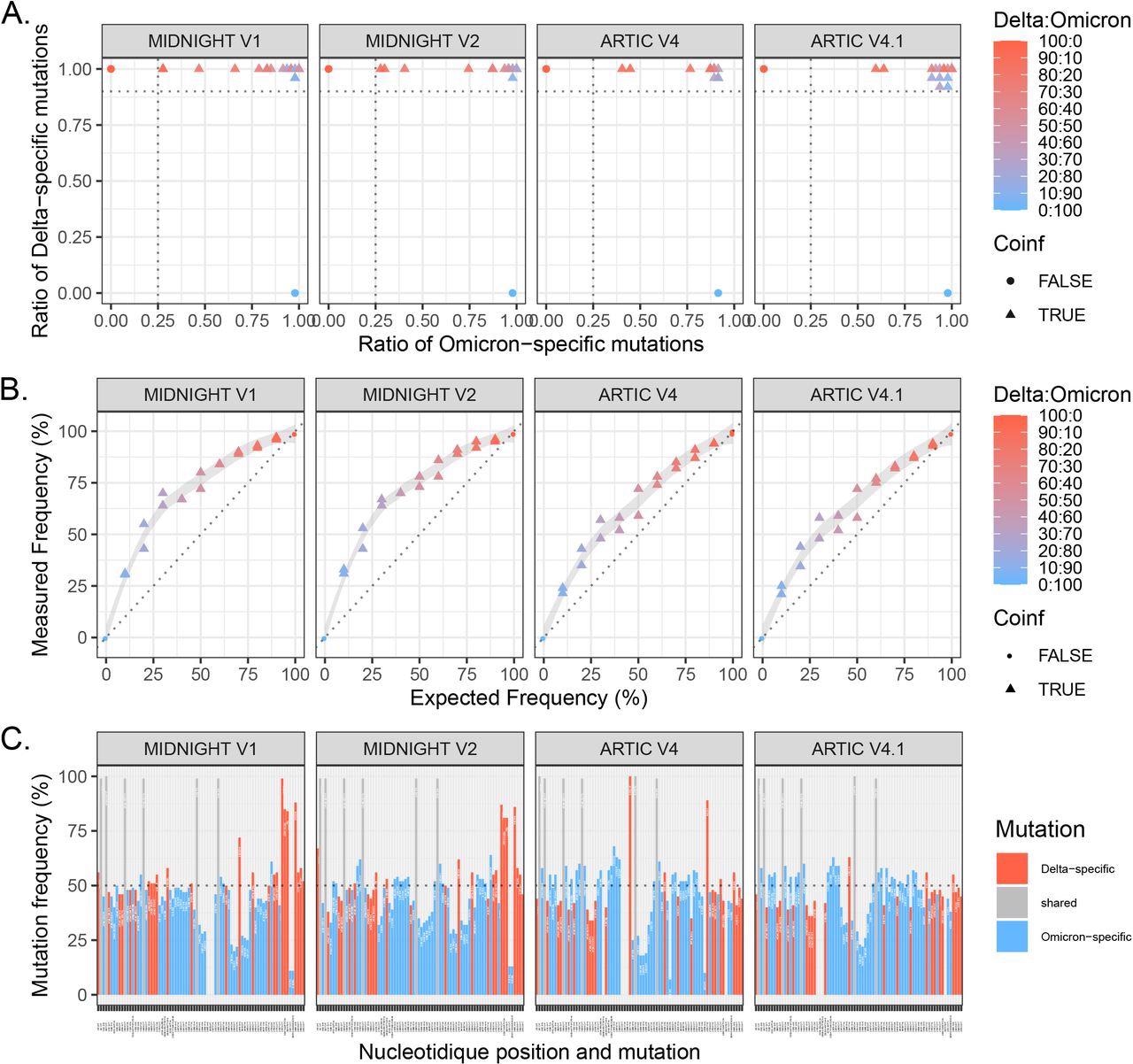
Evaluation of 4 primer sets for WGS of Delta:Omicron mixes: Midnight V1, Midnight V2, ARTIC V4 and ARTIC V4.1.
Eleven mixes of Delta:Omicron isolates with different proportions were extracted and sequenced in duplicate. A. Ratio of specific mutations (as minor or major allele) detected from a list of Delta-specific mutations and Omicron-specific mutations based on covariant.org. Horizontal line (Delta-specific mutation ratio) at 0.9 and vertical line (Omicron-specific mutation ratio) at 0.25 discriminate co-infections from pure isolates. B. Frequency of the Delta variant was measured using the median of covered allele frequencies of Delta-specific mutations and compared with expected relative frequency in each mix. Pure isolates (denoted as 100:0 for Delta and 0:100 for Omicron) are depicted with dots, and Delta:Omicron mixes with triangles. Colors represent the expected frequency of each mix using a red (Delta: 100:0) to blue (Omicron: 0:100) gradient. C. Representation of variant allele frequency of Delta- and Omicron-specific mutations for the mix 20:80. Delta-specific mutations are depicted in red, Omicron-specific mutations in blue and shared mutations are in grey. Horizontal lines at 50% show which mutations are called in the consensus sequence based on the majority rule. Chimeric sequences with both Delta and Omicron mutations were detected for the 4 primer sets.
Of the 53 identified co-infections in the randomly collected samples, 29 were found to be infected with Delta/Omicron (BA.1), while 24 were detected with a co-infection between BA.1 and BA.2. These novel recombinants can be attributed to either the Delta-BA.1 recombinant in a co-infected sample with unbalanced frequencies of Delta- and Omicron-specific mutations, or BA.1-BA.2 recombinant in a co-infected sample with uneven frequencies of BA.1- and BA.2-specific mutations.
Moreover, a prevalence rate of 0.18% for Delta-Omicron (BA.1) co-infections and 0.26% for Omicron lineages (BA.1 and BA.2) co-infections were observed. The detection rate for specific Omicron mutations was below 50% in several Delta: Omicron mixes.
Delta/Omicron co-infections were found to be linked with higher rates of intensive care unit (ICU) admissions than Omicron infections. However, BA.1/BA.2 co-infections did not cause any significant effects.
ICU admission rates were reported to be 1.64%, 4.81%, and 15.38% in Omicron, Delta, and Delta/Omicron patients, respectively, whereas there were no reports of ICU admitted patients in BA.1/BA.2 co-infections. However, these varying clinical spectrums demand investigations that consider the vaccination status of co-infected patients.
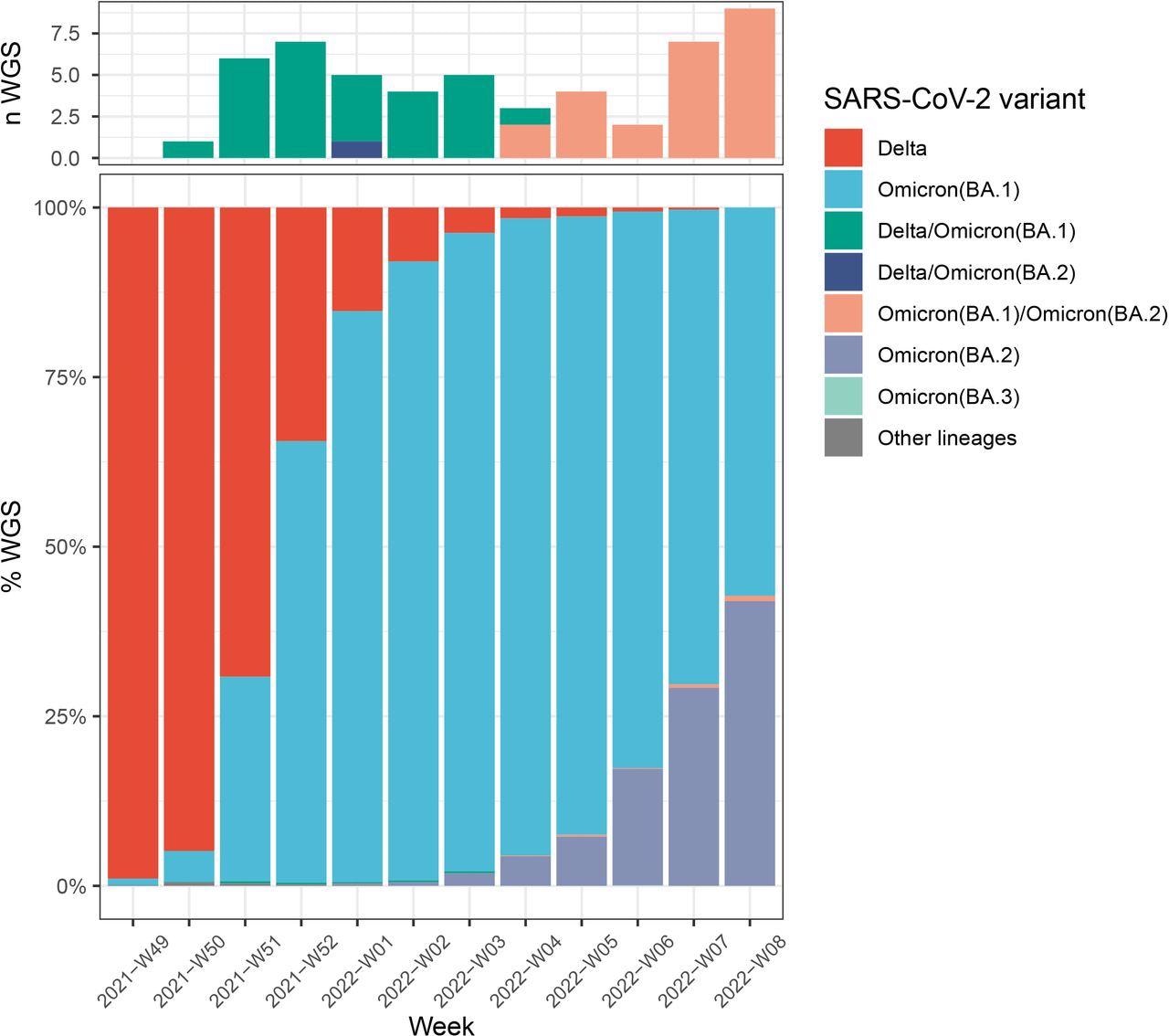
Number of Delta/Omicron (BA.1), Delta/Omicron (BA.2) and BA.1/BA.2 co-infections (A) during Delta, Omicron (BA.1), and Omicron (BA.2) co-circulation in France (B).Colors indicate lineages : Delta (B.1.617.2 and AY.* lineages) in red, Omicron (BA.1) in blue, Omicron (BA.2) in purple, Omicron (BA.3) in light blue, other lineages (including B.1.640.1) in grey. Co-infections between Delta/BA.1 are in green, Delta/BA.2 in dark blue, and BA.1/BA.2 in salmon.
Conclusions
The findings from the current study indicate the need to use suitable experimental and bioinformatics tools for the identification of SARS-CoV-2 co-infections. Although the SARS-CoV-2 co-infections observed in the current study were few, their accurate detection and interpretation are imperative to assess their clinical impact and monitor the risk of potential variants/recombinants.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Bal, A., Simon, B., Destras, G., et al. (2022) Detection and prevalence of SARS-CoV-2 co-infections during the Omicron variant circulation, France, December 2021 – February 2022. medRxiv. doi:10.1101/2022.03.24.22272871. https://www.medrxiv.org/content/10.1101/2022.03.24.22272871v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Bioinformatics, Cell, Cell Line, Coronavirus, Coronavirus Disease COVID-19, covid-19, Gene, Genome, Healthcare, Immune System, Intensive Care, Nasopharyngeal, Omicron, Pandemic, Public Health, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome

Written by
Prajakta Tambe
Prajakta Tambe, Ph.D. worked at Queen’s University Belfast on a project that focused on studying ‘Role of Tregs in Acute Respiratory Distress Syndrome'. Prajakta completed a Ph.D. in August 2020 at Agharkar Research Institute, University of Pune, India. Her work aimed to develop dendrimer-based nanoparticles for the targeted delivery of MCL-1 gene-specific siRNA to bring about apoptosis in breast and prostate cancer cells and in vivo breast cancer xenograft models.
Source: Read Full Article